Resources...
Explore business, technology and compliance resource information
Professional Services
Life Science business consulting services for the Pharmaceutical, Biotechnology and Medical Device industries...
Business Development

Business development services include: program planning, project management and implementation support. Business process development and optimization. Quality Management System assessment, development and implementation. Validation program assessment and implementation. ERP assessment and validation support. Organizational training.
Computer System Validation
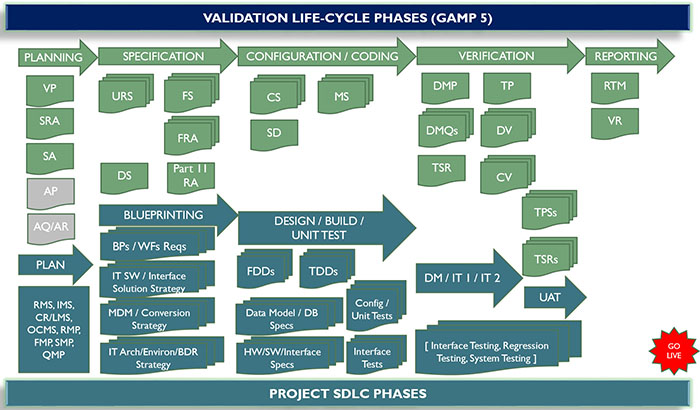
Computer System Validation services for enterprise systems across multiple industries: Pharmaceutical, Biotechnology and Medical Device. IT systems analysis, development and implementation. IT controls assessment and development. Validation services to meet best-practice Good Automated Manufactring Practice (GAMP) and SDLC standards.
Compliance Services

Electronic Records / Electronic Signatures Compliance. FDA cGMP Compliance (21 CFR Part 11, 210, 211, 820, 830). ISO Compliance (9001, 13485, 14971, 27001, 27002). SOX-404 Compliance. GAMP 5. Software Vendor Audits.
Professional Experience
Business Development and Compliance services for the Life Sciences Industry

Expertise: Industries / Business Systems / Compliance Standards
Industry experience: Pharmaceutical 18+ years, Biotechnology 9+ years, Medical Device 13+ years.
Computer System Validation (35+ years): FDA Guidelines, ISPE Commissioning and Qualification, GAMP 4/5, ASTM.
ERP systems (19+ years): SAP R/3 and ECC, JDE EnterpriseOne, Microsoft Dynamics AX, IQMS EnterpriseIQ / 3DS DELMIAworks, and ECI Deacom ERP.
Electronic Record systems (19+ years): EDMS, QMS, GLS, CMS and UDI systems.
IT systems (19+ years): IT networks, RDBMS, BDR systems, and Middleware systems.
Lab systems (18+ years): LIMS, HPLC, GC, UV-Vis, Robotic testing, and Excel.
Clean Utility and Metrology systems (9+ years): DI, WFI, Clean Steam, CIP, SIP, CMMS and BMS systems.
Biotech Process PLC systems (9+ years): Bioreactors, Chromatography, Micro and Ultra-filtration.
FDA regulations (35+ years): FDA 21 CFR Parts 11, 210, 211, 820, and 830.
ISO standards (13+ years): ISO 9001, 13485, 14971, 27001, 27002; and ISO/TR 80002-2.
EU regulations (10+ years): Eudralex Volume 4, Annex 11 and Annex 15.
IT standards (19+ years): Best-practice IT controls, ITIL, ISACA / COBIT, Data Integrity and Management.
Resources...
Business, IT and Compliance resources

Business Resources
American Institute for Economic Research - Research Links
American Institute for Economic Research - Daily Economy Articles
Global PMI Reports - PMI(TM) by IHS Markit
Business Roundtable - CEO Economic Outlook Index
CME Group - Economic Release Calendar
St. Louis Fed - Industrial Production: Total Index
Kansas City Fed - Labor Market Index: Activity Level
New York Fed Empire State Manufacturing Survey
American Injection Molding Institute
IT Resources
Computing Technology Industry Association
Axelos - Global IT Best Practice
International TickITplus Association
ISACA-COBIT: Information Technology - Information Security - Information Assurance
Compliance Resources
ISO - International Organization for Standardization
WHO - World Health Organization
FDA - U.S. Food and Drug Administration
FDA supports the Medical Device Single Audit Program (MDSAP)
European Commission: Eudralex - Volume 4 - GMP Guidelines
Health Canada: Drug and Health Products
Health Canada supports the Medical Device Single Audit Program (MDSAP)
Japan Ministry of Health, Labor and Welfare
Japan Pharmaceutical and Medical Device Agency
Australian Government - Dept. of Health - Therapeutic Goods Administration
New Zealand Medicines and Medical Devices Safety Authority - Medsafe
IMDRF - International Medical Device Regulators Forum
ISPE - International Society for Pharmaceutical Engineering
ANSI - American National Standards Institute
AIAG - Automotive Industry Action Group (IATF 16949)